How to test quality of drugs?
The best method to avoid the risk of psychoactive substances is to not use them at all, but this is not always possible. Drug purity tests are one of the most effective methods of risk reduction. Publicly subsidized programs for anonymously checking the composition of substances operate in various countries such as Austria, the Netherlands, Spain, Germany, and Switzerland (check the map here). They aim to improve citizen safety by enabling substance checking directly before consumption and increasing control over particularly dangerous substances.
As confirmed by numerous scientific studies (e.g., F. Misham et al) and reports from the European Union, checking the composition of substances is an effective method of reaching out to individuals particularly at risk due to drugs who otherwise may not seek support. In this article, we discuss all the most popular methods of testing psychoactive substances (prior to consumption).
Note: neither a negative nor a positive result of substance checking guarantees safety; no substance is 100% safe. If you choose to engage in risky behaviors, make sure you are fully aware of the risks and knowledgeable about how to reduce them. We recommend checking our knowledge base on our Safety subpage.
The most popular methods of checking substance purity include:
- Organoleptic testing
- Solubility
- Boiling point
- Colorimetric reagents
- Diagnostic strips
- UV-Vis (UV-Visible spectroscopy)
- FTIR (Fourier Transform Infrared Spectroscopy)
- TLC (Thin Layer Chromatography)
- GC/MS (Gas Chromatography – Mass Spectrometry)
- LC/MS (Liquid Chromatography – Mass Spectrometry)
- HPLC (High-Performance Liquid Chromatography)
- NMR (Nuclear Magnetic Resonance)
Methods of substance analysis
Qualitative Analysis
- Organoleptic testing
- Solubility
- Boiling point
- Colorimetric reagents
- Diagnostic strips
- TLC (Thin Layer Chromatography)
- FTIR (Fourier Transform Infrared Spectroscopy)
- GC/MS (Gas Chromatography – Mass Spectrometry)
- LC/MS (Liquid Chromatography – Mass Spectrometry)
- HPLC (High-Performance Liquid Chromatography)
- NMR (Nuclear Magnetic Resonance)
Quantitative Analysis
- TLC (Thin Layer Chromatography)
- FTIR (Fourier Transform Infrared Spectroscopy)
- UV-Vis (UV-Visible spectroscopy)
- GC/MS (Gas Chromatography – Mass Spectrometry)
- LC/MS (Liquid Chromatography – Mass Spectrometry)
- HPLC (High-Performance Liquid Chromatography)
- NMR (Nuclear Magnetic Resonance)
Organoleptic Testing
Organoleptic testing, also known as the evaluation of appearance, smell, and taste. For obvious reasons, this method is not precise and is recommended only for negative identification (red flags, not green light).
The most scientifically valuable technique of this type is crystallography, the study of the structure of crystallized substances.
Characteristics of organoleptic testing:
- Low precision
- Low cost
- Simplicity
- High risk of error

Crystallography of cut amphetamine
The primary method of assessing the quality of substances such as amphetamine, MDMA, marijuana, and other psychoactive substances is their visual and olfactory evaluation. Tasting is not recommended due to the risk of pharmacological interaction. If the sample looks or smells inconsistent with expectations, it suggests, even without a more thorough examination, that the substance is not suitable for consumption.
Example warning signs:
- Diverse particle shapes
- Diverse crystal colors
- Intense unexpected odor
- Lack of expected characteristics, e.g., plant material not resembling cannabis flowers
Of course, a positive assessment of appearance and odor does NOT indicate that the sample is safe.
Solubility
Solubility testing involves comparing the behavior of a sample with data available in the scientific literature, e.g., swgdrug.org. This method is also not precise and is recommended only for negative identification (red flags, not green light).
- Low precision
- Low cost
- Simplicity
- High risk of error
- May help purify the substance

Testing substance solubility
Knowing the solubility parameters of the expected substance, you can detect the presence of certain impurities. A good example is caffeine, a common adulterant in powders. Amphetamine, MDMA, or cocaine are relatively well soluble in methanol. However, to dissolve 1 gram of caffeine, you need as much as 75 ml of methanol. Therefore, you can try dissolving a sample (40 mg) in 1 ml of methanol, and if part of the sample remains undissolved, it indicates the presence of an impurity. Naturally, this method is more for detecting impurities than identifying them.
A particularly interesting technique related to solubility is the so-called A/B extraction (Acid/Base). By adding an acid or base, you can change the substance’s form to freebase or salt, simultaneously altering its solubility, which can help filter out unwanted impurities.
Boiling Point
The boiling point test involves heating a substance and observing the point at which it begins to boil. By comparing with data available in scientific literature, e.g., swgdrug.org, you can determine the presence of unexpected ingredients.
Characteristics of boiling point testing:
- Low precision
- Low cost
- Relative simplicity
- High risk of error
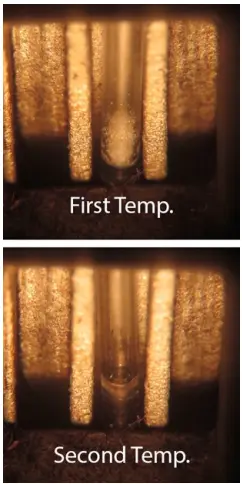
Testing boiling point
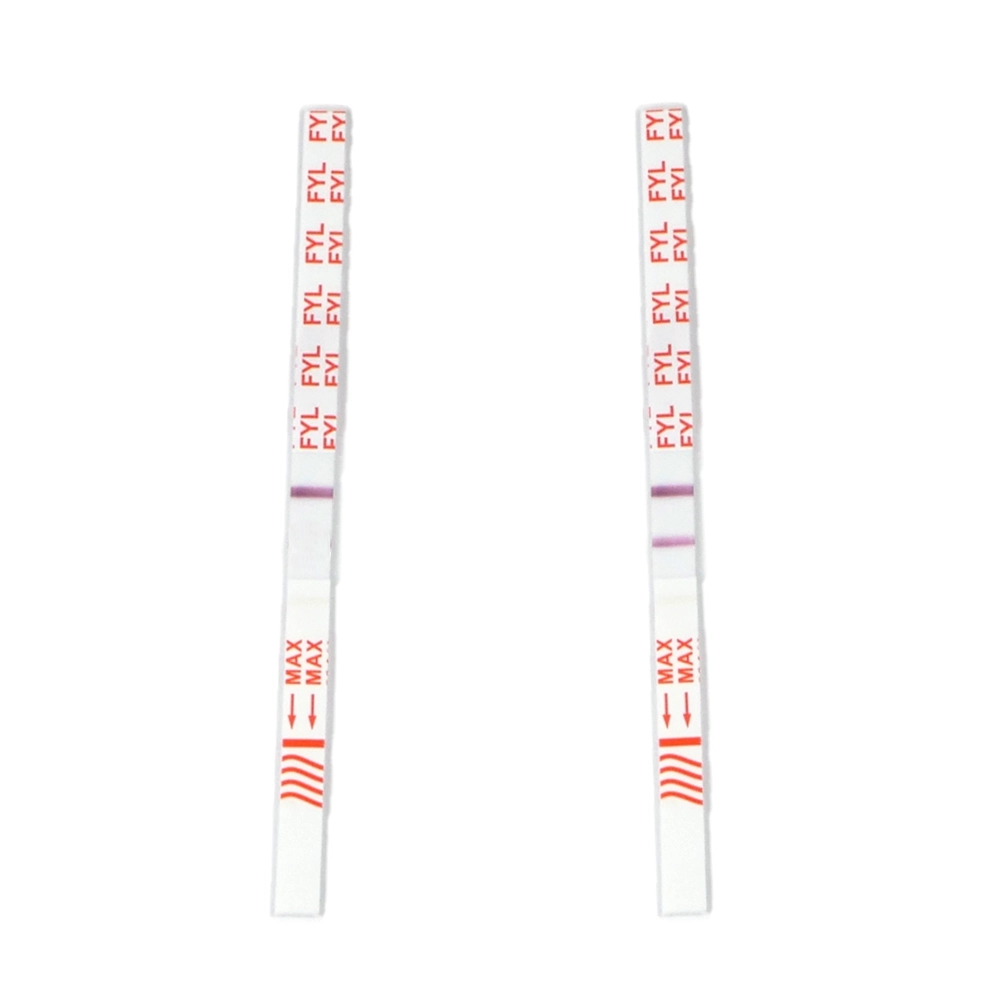
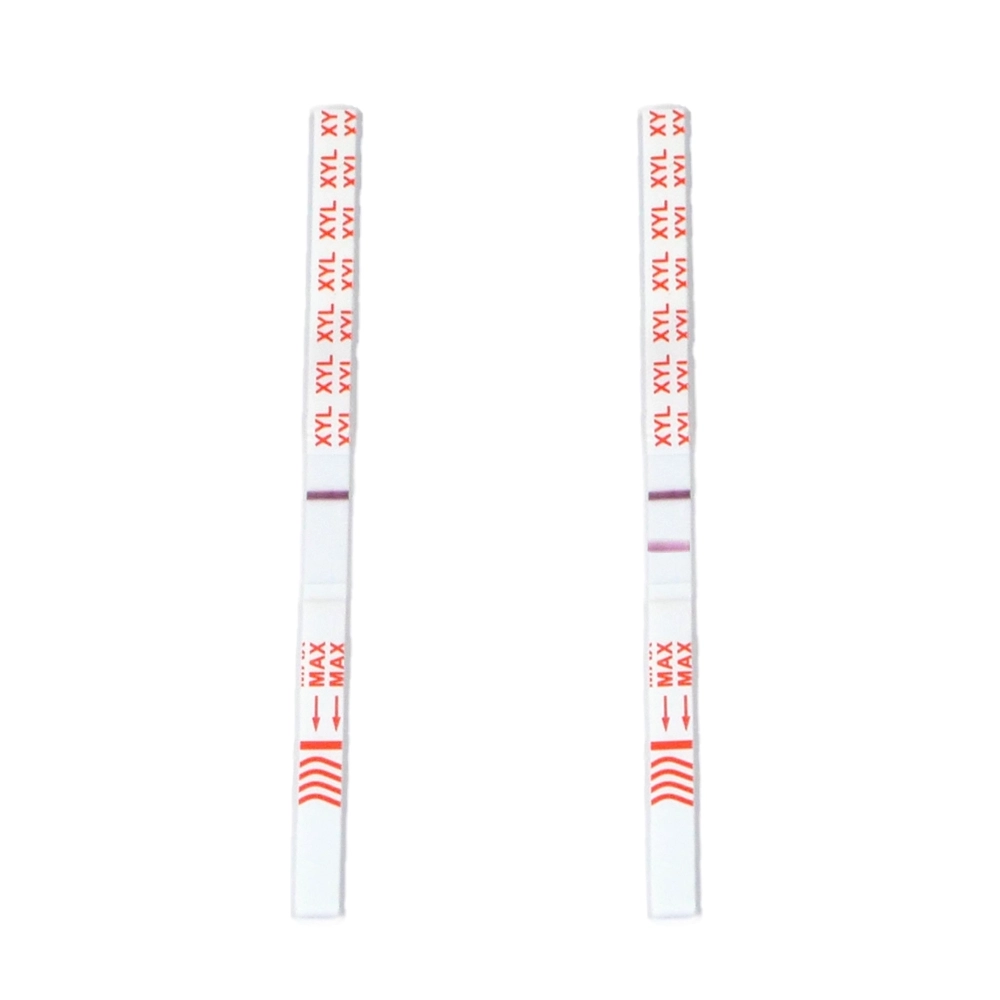
Test Strips
Test strips, traditionally designed for urine testing, can also be used to check substances before consumption. Test strips contain extremely sensitive antibodies that can help exclude the presence of substances, such as fentanyl, in a yes/no manner.
This method is also not precise and is recommended only for negative identification (red flags, not green light).
Characteristics of test strips:
- Limited precision
- Low cost
- Ease of use
- Quick test time

Fentanyl test strips
Immunoenzymatic test strips are diagnostic devices commonly used to detect and quantitatively determine the presence of specific substances in a sample, often in biological fluids such as blood, urine, or saliva. These tests are widely used in medical diagnostics, drug testing, pregnancy testing, and many other applications. They are also applied in forensic contexts or harm reduction to analyze substances before consumption.
Colorimetric Reagents
Colorimetric reagents are simple chemical reagents that change color depending on the added substance.
This method is also recommended for negative identification (red flags, not green light).
Characteristics of colorimetric reagents:
- Limited precision
- Low cost
- Ease of use
- Quick test time

Detecting caffeine in amphetamine
Colorimetric reagents are liquid or crystalline chemical substances that change color depending on the substance with which they come into contact. Since there are significantly more substances than primary colors, it is crucial to use sets of different reagents to increase the precision of identifying the expected substance and exclude some impurities.By using reagents in specially designed kits, such as those offered by PRO Test, especially when combined with techniques like TLC and/or fentanyl strip tests, reliable results can be obtained in home conditions.
There are so-called semi-quantitative colorimetric reagents for checking the concentration of substances. However, the interpretation of their results is generally highly subjective and not reliable. In the PRO Test offer, you can find reagents that, although qualitative tests, have an additional semi-quantitative application. Semi-quantitative tests help assess the concentration in a relative way, i.e., in relation to a sample or reference scale.
PRO Test semi-quantitative reagents:
- Cannabis (4-AP) – checks whether the sample mainly contains CBD or THC
- Hofmann – helps compare the amount of LSD
- Zimmermann – helps compare the amount of CBD
Thin Layer Chromatography (TLC)
Thin Layer Chromatography allows the separation of a sample into individual substances. Each substance is visible as a separate “spot.” TLC can assess concentration because often a larger “spot” indicates a higher concentration.
Characteristics of Thin Layer Chromatography:
- Low cost per test
- Relatively simple to use
- Detects the number and quantity of substances
- Does not identify substances

TLC Cannabinoids test
It can identify the active components of a very wide range of substances, differentiate between derivatives of the same group, and detect the most common adulterants. The obtained results separate active substances in the sample, but it is not always possible to determine purity or concentration. To identify the detected substances, additional colorimetric reagents are required.
The TLC method is entirely dependent on the “test liquid” used for the analysis, also called the eluent or “mobile phase.” In some cases, it is able to detect even isomers of the same substance.
Fourier Transform Infrared Spectroscopy (FTIR) is the most cost-effective among advanced methods of substance analysis. It can be utilized with minimal training and is applied, among others, by getyourdrugstested.com.
Features of FTIR:
- relatively high device cost
- often relies on a paid subscription to a database
- relatively simple to use
- suitable for samples with 1-2 components
- does not detect substances below 5% concentration

FTIR device
Fourier Transform Infrared Spectroscopy (FTIR) is an analytical technique used in chemical analysis for the identification and quantitative determination of chemical compounds based on their absorption of infrared radiation. FTIR provides information about the functional groups present in the sample, facilitating the identification of molecular structures. It is valued for its sensitivity, speed, and ability to provide detailed information about molecular structures, making it an essential tool in modern analytical chemistry.
UV-Vis
UV-Visible Spectroscopy is a popular method of analysis. Similar to FTIR, it does not destroy the tested sample. It is often used in conjunction with methods for substance identification.
Features of UV-Vis:
- Relatively high equipment cost
- Detects the quantity of substances
- Does not differentiate isomers of the same substance

UV-Vis aparatus
Analytical technique used for the quantitative determination of unmixed substances. For instance, in the laboratory energycontrol-international.org, it is most commonly used for the quantitative analysis of MDMA/Ecstasy, 4-MMC/Mephedrone, 3-MMC/Metaphedrone, and 2-MMC. It is also applied to substances such as 2C-B/Nexus, MDA, and 6-APB. This is a quantitative technique used for substances requiring more precise dosage control to achieve desired effects and reduce risk.
UV-Vis analysis, or UV-Visible spectroscopy, is a widely used analytical technique measuring the absorption of ultraviolet (UV) and visible light by a substance. This technique is used to obtain information about the electronic structure of molecules and for the quantitative determination of substance concentration in a sample. UV-Vis spectroscopy is a versatile and widely used technique due to its simplicity, speed, and sensitivity. It is a valuable tool in various scientific fields, including chemistry, biochemistry, environmental sciences, and materials science.
GC/MS
Main laboratory method for substance analysis, often used as a screening method to identify a compound or compounds in a sample.
Features of GC/MS:
- High equipment cost
- Detects the type and quantity of substances
- Distinguishes between multiple isomers
- Not suitable for thermally sensitive substances

GC/MS devices
GC/MS is used for both qualitative and quantitative analysis of major substances, such as cocaine, amphetamine, methamphetamine, sedatives, ketamine and its impurities, as well as other substances. It enables the identification of NPS (new psychoactive substances) and allows for the quantitative determination of most substances, even when mixed.
It allows differentiation of positional isomers, such as 4-MMC and 3-MMC, through retention time, except for fluoroderivatives (2,3,4-FMA; 2,4-FA; 2,3,4-FEA).
LC/MS
Next to GC/MS, LC/MS is the second most popular laboratory method for substance analysis. It is commonly used for psychoactive substances, especially for LSD and fentanyl.
Characteristics of LC/MS:
- High cost of equipment
- High precision
- Detects the type and quantity of substances
- Does not differentiate isomers of the same substance
- Suitable for thermally sensitive substances

LC/MS devices
Liquid Chromatography with Mass Spectrometry (LC/MS) is an effective analytical method in which a sample is separated on a liquid chromatography column and then identified using mass spectrometry. This technique is used for the analysis of diverse substances, both organic and inorganic.
LC/MS is valued for its ability to quantitatively determine compounds in samples with complex chemical compositions, making it widely used in biological, pharmaceutical, and environmental research.
HPLC
High-Performance Liquid Chromatography (HPLC) is widely considered the best practical method for analyzing substances. It is used by organizations like Energy Control for assessing the concentration of LSD in blotters and drops.
Features of HPLC:
- High equipment cost
- Very high accuracy
- Detects the number and quantity of substances
- Requires reference samples for identification
- Suitable for thermally sensitive substances

HPLC devices
High-Performance Liquid Chromatography (HPLC) is a precise analytical method designed for the separation and analysis of chemical compounds in a liquid sample. In HPLC, the sample is passed through a chromatographic column under very high pressure, allowing for effective component separation.
This type of chromatography is particularly effective in the analysis of organic substances, such as drugs, nucleic acids, or phenolic compounds. HPLC is widely used in analytical and industrial laboratories for the precise determination of concentrations of various substances in samples.
NMR
Nuclear Magnetic Resonance (NMR) is the most advanced, time-consuming, challenging, and expensive method for analyzing substances. It is also the most accurate of all. The cost of analyzing one sample can amount to several thousand złoty.
Characteristics of NMR:
- Enormous device cost
- Highest possible accuracy
- Most challenging to use
- Often requires an entire room for setup

NMR devices
Nuclear Magnetic Resonance (NMR) analysis is a powerful and versatile analytical technique used to study the structure and dynamics of molecules. It provides detailed information about the nuclear environment of specific atoms within a molecule, offering insights into molecular structures, molecular dynamics, and interactions. NMR is widely used in chemistry, biochemistry, and related scientific fields.
Regarding costs, NMR instruments are generally very expensive to purchase, operate, and maintain. The complexity of the technology, the need for a powerful magnet, and specialized knowledge contribute to high costs. Additionally, sample preparation costs and the time required for data acquisition and analysis can also impact expenses.
While NMR is an incredibly valuable and versatile analytical tool, the choice of the “best” technique depends on specific analytical goals and the nature of the sample under investigation. The perceived costs of NMR are often justified by its capabilities and the detailed information it provides about molecular structures and dynamics.
Summary
After reading this article, you might be wondering if the existence of sophisticated analysis methods like NMR or HPLC is an argument against simpler ones. Well, no, each method has its application. Knowing their limitations, you can successfully obtain a lot of information using straightforward tools.
TLC and colorimetric reagents, which do not require specialized knowledge, allow for very affordable checking of the purity of drugs. Their results are reliable when used according to the recommendations of PRO Test kits. By using specially designed test kits, you can have a high level of confidence in the results.
Are simple analytical methods infallible? Of course not, but no test result guarantees safety. Always be aware that a substance may contain a new, challenging-to-detect component. However, let’s not forget that no substance is 100% safe.
Start testing your substances now:
No test kit results can guarantee if a substance is safe. No substance is 100% safe.